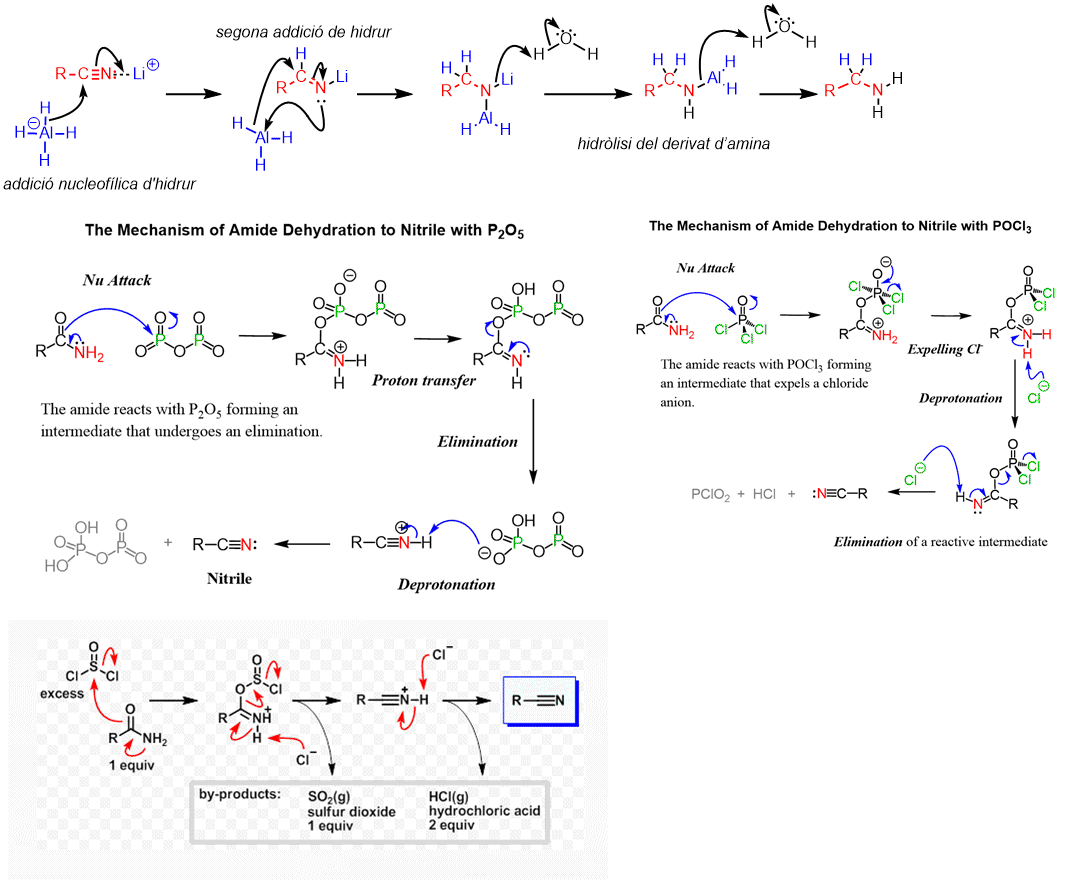
Dehydration of amides to nitriles
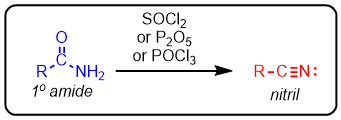
Primary amides can be dehydrated to nitriles in the presence of SOCl2 or P2O5
Primary amides can be converted to nitriles by strong dehydrating agents such as SOCl2, P2O5, and POCl3:
Mechanism
- The reaction starts with a nucleophilic attack of the C=O oxygen which converts into a good leaving and then eliminated in the following steps:
- xxxx
- he last step is irreversible formed by loss of good leaving groups and entropy factors.
The dehydration of mechanism of amides by POCl3 is very similar to the one with SOCl2. The C=O oxygen is converted into a good leaving group and eliminated in a later step:
P2O5 is another powerful dehydration agent which converts amides to nitriles by a similar mechanism: