LiAlH4 reducción of amides to amines
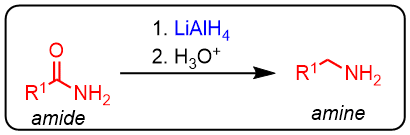
The amides can be reduced to amines using a hydride source, such as LiAlH4.
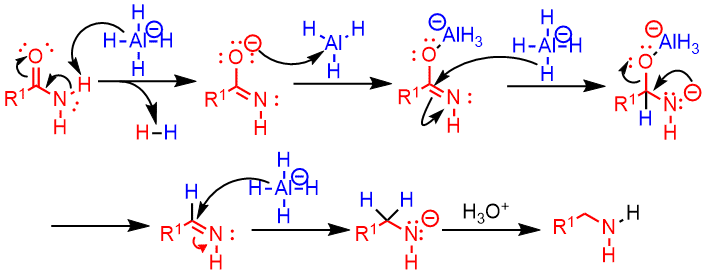
Mechanism
- The hydride attacks, through a nucleophilic addition, the hydrogen of the amide and releases molecular hydrogen.
- The oxygen attacks the aluminum and a good leaving group is formed which will be removed.
- It forms an imine that will be reduced by the aluminum hydride.
- Finally, the acid in the middle will protonate the intermediate to give rise to the final amine.